A Better Trial Experience for Sites, Patients,
and Study Teams. And Better Results.
more likely to hit recruitment targets vs. sites not using Longboat
fewer deviations vs sites not using Longboat
fewer logins required to support critical workflows for sites
Centralize, connect, and simplify
your study with an intuitive
technology that sites love using
-
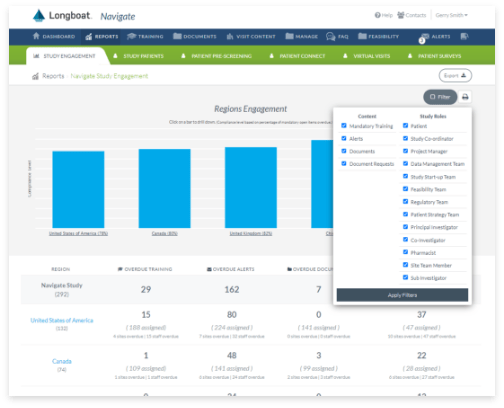
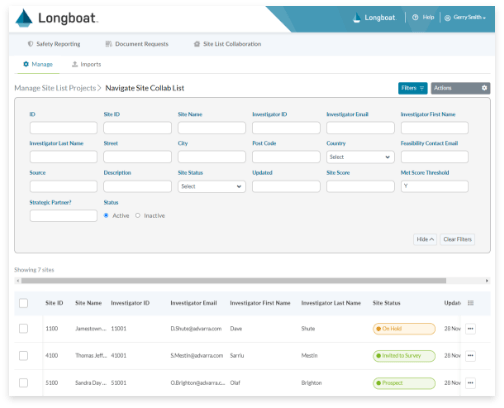
Global Oversight Dashboard and Analytics
Complete global visibility for real-time reporting across all site and patient activities assigned, in-progress, and completed on your trial or across your portfolio.

-
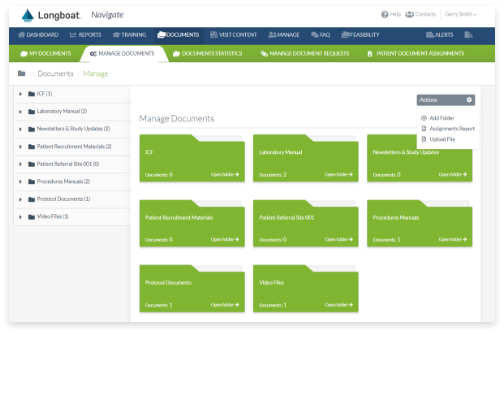
Secure Document Exchange
Accelerate study startup via end-to-end integration and automated, seamless exchange of research documents between sites, sponsors, and CROs.

-
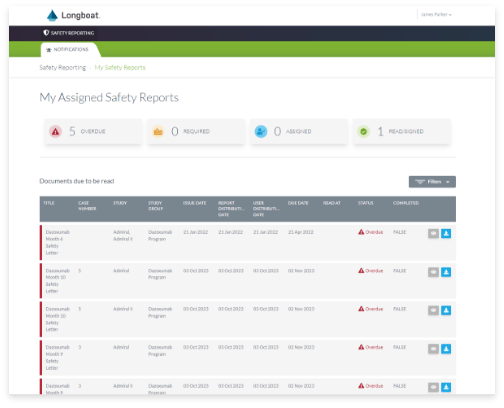
Safety Letter Management
Simply and effectively distribute safety reports to clinical investigators with visibility into status at the site, region, or global level across your clinical program.

-
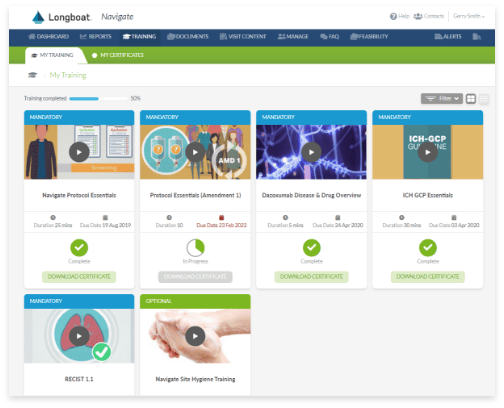
Learning Management System
Support site staff with study-specific training, including task- and event-based training deployment, assessment and certification, and oversight into training completion.

-
Site Feasibility
Gather critical information about potential site partners with a workflow tool to combine multiple site lists, route for review/approval, and track site status.

-
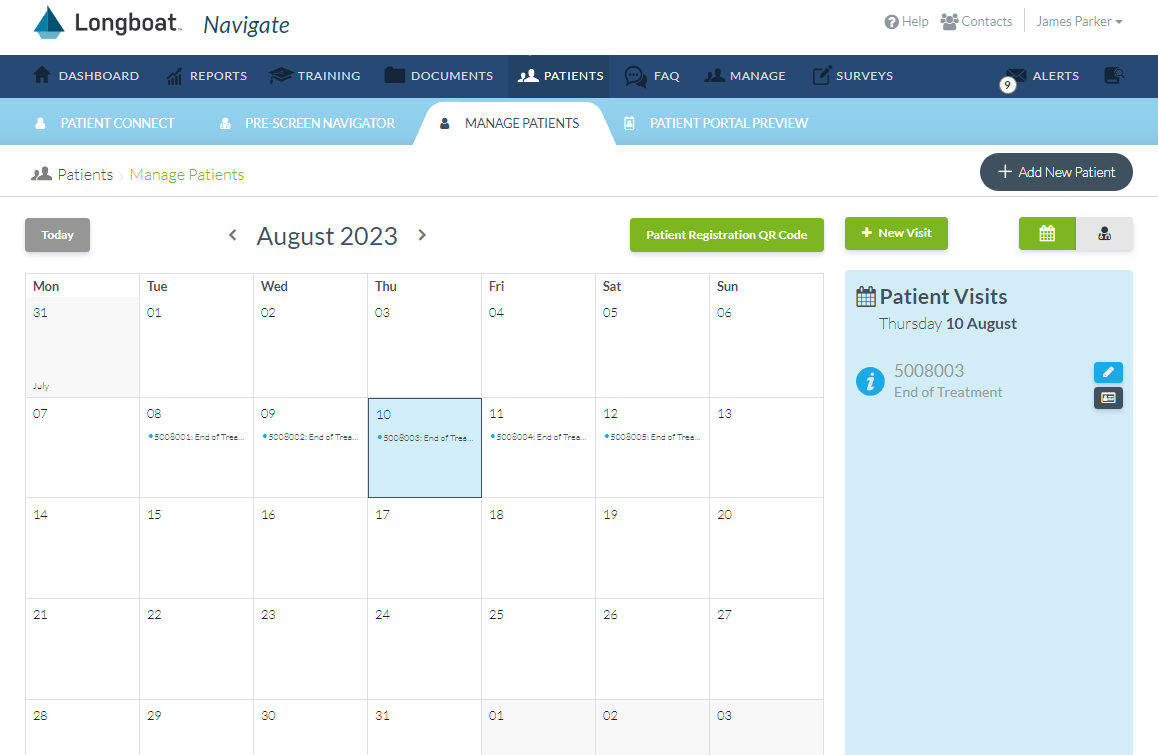
Site Engagement
Centralize engagement and oversight of site operations while providing intuitive resources for prescreening, consent, visit guidance, and visit calculations to increase compliance.

-
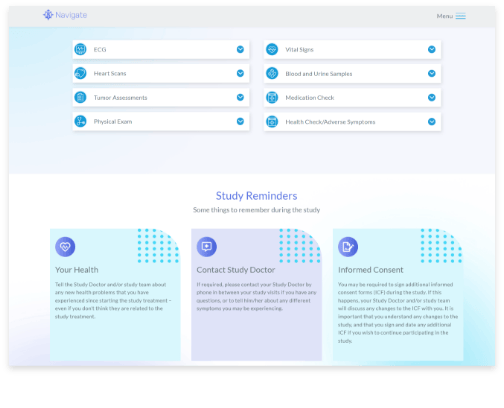
Patient Engagement and Referral Management
Support participants on their study journey from recruitment through to trial close-out with information and resources they need to be informed, compliant, and retained.


Unifying Global Research Teams
Across 71 Countries
Research Personnel
Sites
Study Participants
Why Longboat?
The clinical trial experience has to change.
Research sites are navigating too much disconnected technology on top of industry challenges like increasingly complex protocols and staffing. In a recent survey conducted by Advarra, sites reported sponsor technology training as the most burdensome activity in study startup, as well as reporting the use of six or more logins required per study. Learn how Longboat’s centralized, connected, and simplified approach eases these burdens to enable an improved, efficient, and compliant experience for all.
Sites want central, easy access to study technology and resources
81% of sites say using their own site credentials would be valuable when accessing various trial conduct platforms.
Centralized Access and Accepts Org Credentials
Longboat centralizes access to additional study platforms and reduces the number of logins by accepting organizational credentials to log in. It also provides a comprehensive view of all study activities and necessary actions.
Senior Patient Engagement Specialist, Top 3 CRO
Sites want the choice to use their own technology
Over 70% of sites say connecting their eReg system to the sponsor or CRO document exchange technology would be extremely or very valuable.
Preferred Technology for Every Type of Site
Longboat supports integration with Advarra eReg site customers and provides full eISF functionality for tech-naive sites who may not have regulatory management resources in-house. Allowing every site to work in the system of their choice.
Associate Dean for Clinical Research, Large Academic Medical Center
Thoughtful, complimentary site engagement tools are helpful
80% of sites rated engagement tools such as visit essentials, quick links, visit calculators, and protocol text search as extremely or very useful.
Intuitive and Quality User Experience
Longboat’s clear and friendly interface makes it easy to stay up-to-date on all study needs and take necessary action, without having to leave the system.
Medical Director,
Top 5 CRO
Integration Capabilities
Longboat is connected to the top 15 systems most used by sponsors and CROs to facilitate trials. Below are the most impactful integrations that improve efficiency, compliance, and collaboration across stakeholders.
Sponsor eTMF
Automates IRB-related and site essential documents and data integrated from the Advarra IRB and Longboat to the sponsor or CRO eTMF mapped directly to study, study country, and site level.
Site Technology Integrations
Exchange essential documents between sponsor/CRO eTMF, Longboat, and site eISF systems.
Patient Recruitment Tools
Automatic and direct feed of third party patient recruitment company referrals into Longboat site engagement tools for outreach, additional screening, and enrollment and central recruitment oversight.
Interactive Response Technology (IRT)
System Randomization number sent from IRT provider to Longboat in order to track enrollment in the platform.